1. Hybridization is the coming together, or binding, of two genetic sequences. The binding occurs because of the hydrogen bonds [pink] between base pairs. Between a A base and a T base, there are two hydrogen bonds; between a C base and a G base, there are three hydrogen bonds.

2. When making use of hybridization in the laboratory, DNA must first be denatured, usually by using heat or chemicals. Denaturing is a process by which the hydrogen bonds of the original double-stranded DNA are broken, leaving a single strand of DNA whose bases are available for hydrogen bonding.

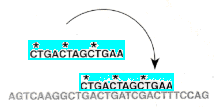
3. Once the DNA has been denatured, a single-stranded radioactive probe [light blue] can be used to see if the denatured DNA contains a sequence similar to that on the probe. The denatured DNA is put into a plastic bag along with the probe and some saline liquid; the bag is then shaken to allow sloshing. If the probe finds a fit, it will bind to the DNA.
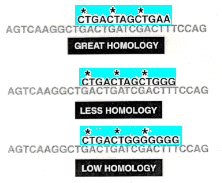
4. The fit of the probe to the DNA does not have to be exact. Sequences of varying homology can stick to the DNA even if the fit is poor; the poorer the fit, the fewer the hydrogen bonds between the probe [light blue] and the denatured DNA. The ability of low-homology probes to still bind to DNA can be manipulated through varying the temperature of the hybridization reaction environment, or by varying the amount of salt in the sloshing mixture.